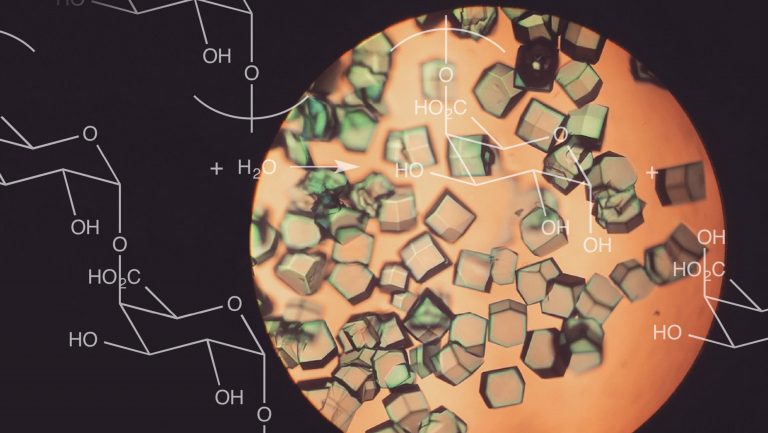
In many winemaking circles the use of enzymes—and additives in general—is controversial. But whether you love them or hate them, an understanding of what they do gives insight into the nature of wine. Enological enzymes are a group of enzymes that are used by many winemakers to aid in extraction, to enhance aromas, and to block malolactic fermentation (MLF). Wine aside, enzymes make up an enormous category of compounds that are essential to life. Most are proteins, although their defining characteristic is simply that they speed particular chemical processes.
Grapes and fermentations are inherently rich in their own naturally occurring enzymes, derived from the grapes themselves or the bacteria, yeasts, and fungi on their surfaces. The production of alcohol by yeast is actually an enzymatic process: Sugar is converted little by little into alcohol by numerous enzymes within yeast. However, many naturally occurring enzymes are inhibited by must pH and sulfur dioxide (SO2) levels, making them inefficient at enacting any significant changes in a fermentation—or in a wine’s character.
Originally used in the production of fruit juice in the 1950s, to increase juice yield and improve clarification, commercial enzymes were adopted by the wine industry worldwide in the 1970s. Today commercial enzymes serve various practical functions in winemaking, from saving time and space to increasing must yield.

Don’t miss the latest drinks industry news and insights. Sign up for our award-winning newsletters and get insider intel, resources, and trends delivered to your inbox every week.
Extraction, Clarification, and Filtration
The names of the majority of enzymes end in “-ase,” which makes them easy to identify. The best-known group of enzymes are pectolytic—or pectic—enzymes, such as polygalacturonase, pectin lyase, and pectin methyl esterase. These enzymes are derived from various fungi, primarily Aspergillus, Trichoderma, and Rhizopus, and they are used to break down pectins.
Pectins, and related compounds, are largely responsible for cell structure and cell binding in fruits. Pectolytic enzymes break down pectins at different points of connection, thereby breaking down grape cells, which allows for a wide range of applications at different times during winemaking. For example, in both white and red wines, pectolytic enzymes increase juice yield from grapes and the quantity of aromatic precursors in a must in both free and press runs. (Aromatic precursors refers to aromatic compounds, like terpenes, that remain bound to other molecules in grapes and are not aromatic until they’re liberated during fermentation.)

After pressing white grapes, winemakers almost always settle the must overnight, though pectolytic enzymes can drastically speed this process and lead to a more complete sedimentation, which further increases must yield. Lauren Barrett, a winemaking specialist at Enartis, a leading supplier of enological products and analytical services headquartered in Windsor, California, points out that researchers have found an increase in must yield of 15 percent in reds and just over 7 percent in whites when pectolytic enzymes are used.
Some wineries filter white musts before fermentation, and a more complete sedimentation also makes the filtration of white musts easier, as well as up to three times faster, according to researchers at Goce Delchev University in Štip, Macedonia. This saves time, space, and wear on expensive filters. A more complete settling of solids also helps remove the majority of spoilage bacteria, which tend to cling to solid particles in fermentations. Cleaner white wine musts also lead to more ester production and fruitier wines.
In red musts, pectolytic enzymes break down pectins to release color and tannin much faster, allowing shorter macerations to achieve desired levels of extraction. Sarah Cabot, the Oregon winemaker for Precept Wine, a large producer of wines from several states that’s headquartered in Seattle, explains that she has a limited number of tanks and a lot more fruit than the tank capacity will accommodate. “I use [enzymes] purely for logistical reasons—I have to free up my tanks,” she says. “In Oregon, everything usually comes in over a two-week period, so I use enzymes on my larger lots and press off sweet [sweet, meaning before all of the sugar has been converted to alcohol] after a few days.”
Cabot has done trials comparing fermentations with shorter, enzyme-assisted macerations and longer, unassisted macerations, and she has found little difference. “It’s like putting ice in a drink because you don’t have access to a fridge,” she says—“speeding up a process that would happen naturally if I had more time.” Cabot adds that she doesn’t use enzymes in her smaller lots because she’s not rushed to free up tank space and feels it would be a waste of money.

Winemakers like Brian Loring, who is also the owner of Loring Wine Company in Lompoc, California, may use pectolytic enzymes for other practical reasons. “We use a small amount of enzymes to help break down skins during cold soak,” he says, “so that we can get more accurate sugar readings prior to fermentation.”
Enzyme companies usually offer numerous blends, often referred to as cocktails, whose proportions and combinations of various pectic enzymes differ. The various blends offer differing activities—while some are focused on the aforementioned activities, others are aimed at speeding up lees autolysis, performing clarification by flotation, and aiding ease of filtration for botrytis-affected wines.
Blocking Lactic Acid Bacteria and MLF
Lysozyme is an antimicrobial enzyme that occurs widely in nature, notably in human tears. In wine, it’s commonly used to block MLF. Lysozyme degrades the cell walls of lactic acid bacteria—among other types of bacteria—making this enzyme an effective tool to prevent MLF for up to a few years after bottling. Commercially available lysozyme is extracted from egg whites, where it’s found in high concentrations.
Lysozyme may also allow winemakers to use less SO2 in white, low pH wines that do not undergo MLF. In those wines, it’s standard practice for winemakers to add 50 parts per million (ppm) of SO2 to must before fermentation to block MLF, after which further additions of SO2 are made until bottling to maintain blockage, when sterile filtration is typically carried out to remove malolactic bacteria. However, lower pH wines require much less SO2 to reach the industry standard for microbial protection. By adding lysozyme, winemakers can still block MLF for these lower pH wines while making smaller SO2 additions. Adding SO2—especially at the levels commonly used for non-MLF white wines—is disfavored by some winemakers who believe it can hinder a wine’s ability to express its vineyard origins, or who may be concerned that it will interfere with the wine’s quality.
At least one study found that lysozyme use had no effect on wine aroma or palate during the study’s six-month post-fermentation observation period. If lysozyme doesn’t exert a noticeable influence on flavor, it may be an option worth exploring for producers of non-MLF white wines who prefer to avoid using SO2. Though studies on taste are often disputable, lysozyme is believed to interact with far fewer wine constituents than does SO2.
The Science of Winemaking Yeasts
Wine professionals discuss the impact of wild and commercial strains on fermentations
Liberating Aromatic Compounds
Glycosidase enzymes are used to unbind aroma compounds, specifically terpenes. In grapes, aroma compounds are primarily glycosylated, or bound to sugar molecules. When bound, these aroma precursors are not volatile and therefore not aromatic. During fermentation, varying levels of grape aromatics are unbound and become volatile and aromatic. The extent of aroma liberation depends on different factors in fermentation, but an important one is the level of glycosidase produced by yeast. The aromatic intensity in wines from terpene-rich grapes, like Muscat and Viognier, can be elevated by the action of glycosidase enzymes, most notably beta-glucosidase. Certain yeast strains produce larger quantities of this enzyme than others; however, winemakers can also add commercial glycosidase enzymes to achieve a similar—and possibly even more intense—aromatics-boosting effect.
While enological enzymes are generally associated with large commercial wineries, they have numerous uses during production, which is why many small wineries and home winemakers also choose to use them. Enzyme technology is still evolving, as this is a relatively young field, and may lead to a myriad of new applications in the future. “The enzyme activity found in many wine microorganisms,” says Barrett, “especially some non-Saccharomyces yeasts, displays desirable [effects] that could further be optimized [for use] by winemakers.”
Although some may not like the idea of additives in a wine, an understanding of how enzymes are used in winemaking—what they do, the chemistry involved, and the structures and compounds the enzymes interact with—leads to a fuller understanding of grapes and wine development.

Dispatch
Sign up for our award-winning newsletter
Don’t miss the latest drinks industry news and insights—delivered to your inbox every week.
Alex Russan, based in Los Angeles, is a former winemaker, importer, and sherry bottler. He writes about viticulture, enology, tasting and the nature of wine